
ANALYTICAL METHOD DEVELOPMENT
No process or product can move forward without robust, validated methods that reliably measure purity, potency, stability, and safety. At Lupin Manufacturing Solutions, we specialize in Analytical Method Development services that support molecules from discovery through commercialization. Our approach is phase-appropriate: methods are designed to be fit for their intended use, whether it is an early tox batch, a clinical trial supply, or a fully validated commercial API. With deep expertise in complex molecules, impurities, and regulatory compliance, we ensure your development pathway is supported by data that stands up to global scrutiny.
Why Analytical Method Development Matters
Poorly designed methods can delay or derail development. Inadequate selectivity, insufficient sensitivity, or lack of robustness often leads to:
- Regulatory rejection of data packages
- Batch release delays
- Inconsistent results across labs and scales
- Unexpected impurities missed until late in development
At LMS, we address these risks by designing stability-indicating, reproducible, and validated methods aligned with ICH and pharmacopeia standards.
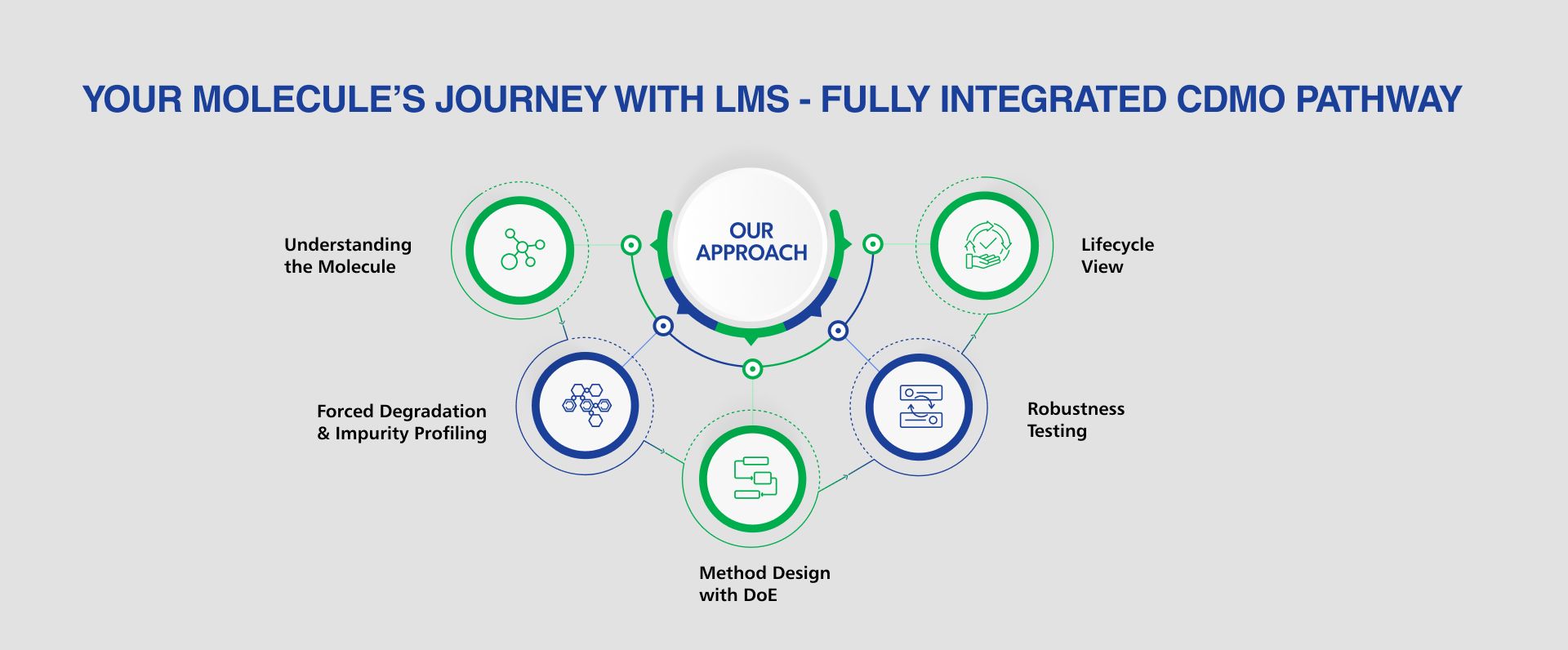
What Do We Offer:
- Assay methods for potency determination
- Related substances/impurity profiling
- Residual solvents and heavy metals analysis
- Chiral methods for enantiomeric purity
- Polymorph and solid-state analysis
- Validation in line with ICH Q2(R2) guidelines
- Accuracy, precision, specificity, detection limits, linearity, and robustness
- Seamless method transfer between LMS labs and client facilities with bridging studies
- Forced degradation studies to establish degradation pathways
- Analytical methods tailored to capture degradation products
- Long-term and accelerated stability testing
- Extractables and Leachables profiling for APIs and intermediates
- Nitrosamine and elemental impurities testing using advanced platforms
- Prep HPLC and chiral purification to generate analytical standards
- Reference standard qualification and lifecycle management
WHY LMS ?
- Integrated development model Analytical scientists embedded alongside process chemists and engineers
- Global regulatory experience Methods designed with FDA and ICH expectations in mind
- Expertise in complex APIs HPAPIs, hormones, macrolides, iron colloids, and controlled substances
- Speed and reliability Rapid turnaround times without compromising quality
- Lifecycle support From feasibility methods to full validation and post-approval changes

