DRUG SUBSTANCE
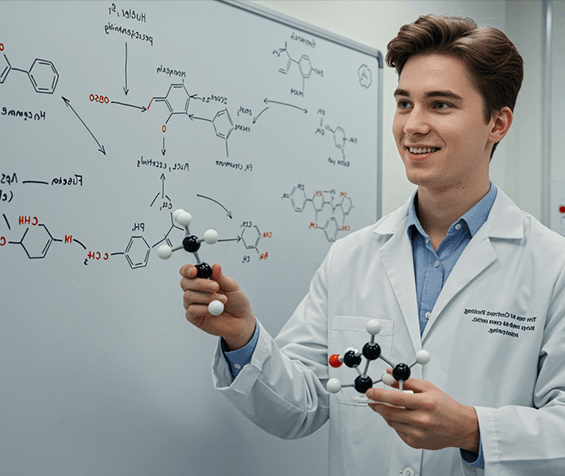
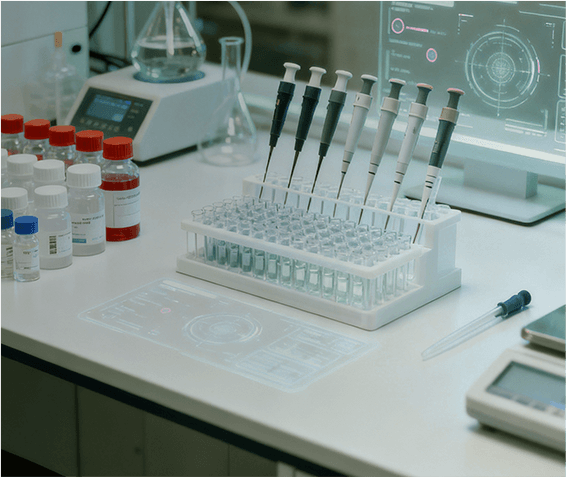
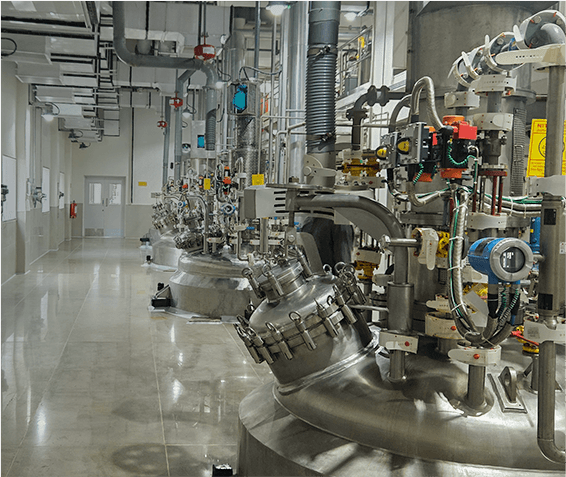
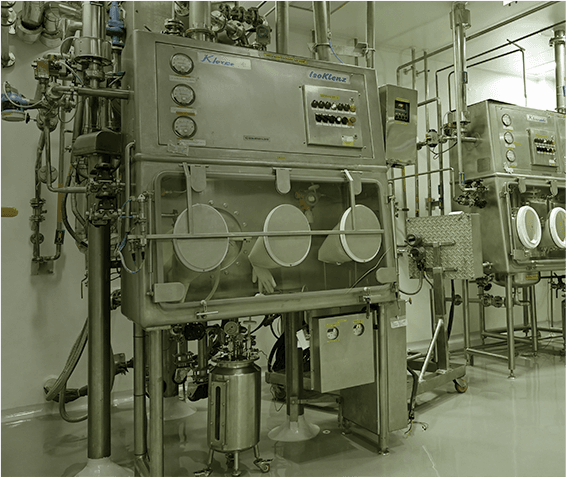
Lupin Manufacturing Solutions (LMS) offers end-to-end drug substance CDMO solutions, supporting projects from early development through clinical and commercial manufacturing. Our capabilities span route scouting, process development and optimization, scale-up, technology transfer, and large-scale GMP manufacturing, backed by strong analytical and quality systems.
We specialize in complex small molecules, high-potency APIs, and advanced chemistries, supported by robust containment and compliance infrastructure. With regulatory-aligned processes, reliable supply continuity, and global quality standards, LMS helps partners reduce risk and accelerate timelines. Backed by Lupin’s proven manufacturing legacy, we deliver safe, scalable, and scientifically rigorous drug substance solutions for global markets.