
CLINICAL SUPPLY
Clinical trials are the critical bridge between scientific discovery and patient access. At this stage, a reliable supply of GMP-compliant APIs is essential to maintain study integrity, meet regulatory standards, and avoid costly delays. At Lupin Manufacturing Solutions (LMS), we specialize in Clinical Supply services that ensure APIs for Phase I, II, and III clinical trials are manufactured, controlled, and delivered on time. With globally inspected facilities, a deep legacy of regulatory excellence, and robust project management, we enable pharma innovators and biotech companies to conduct trials with confidence.
Our Clinical Supply Services
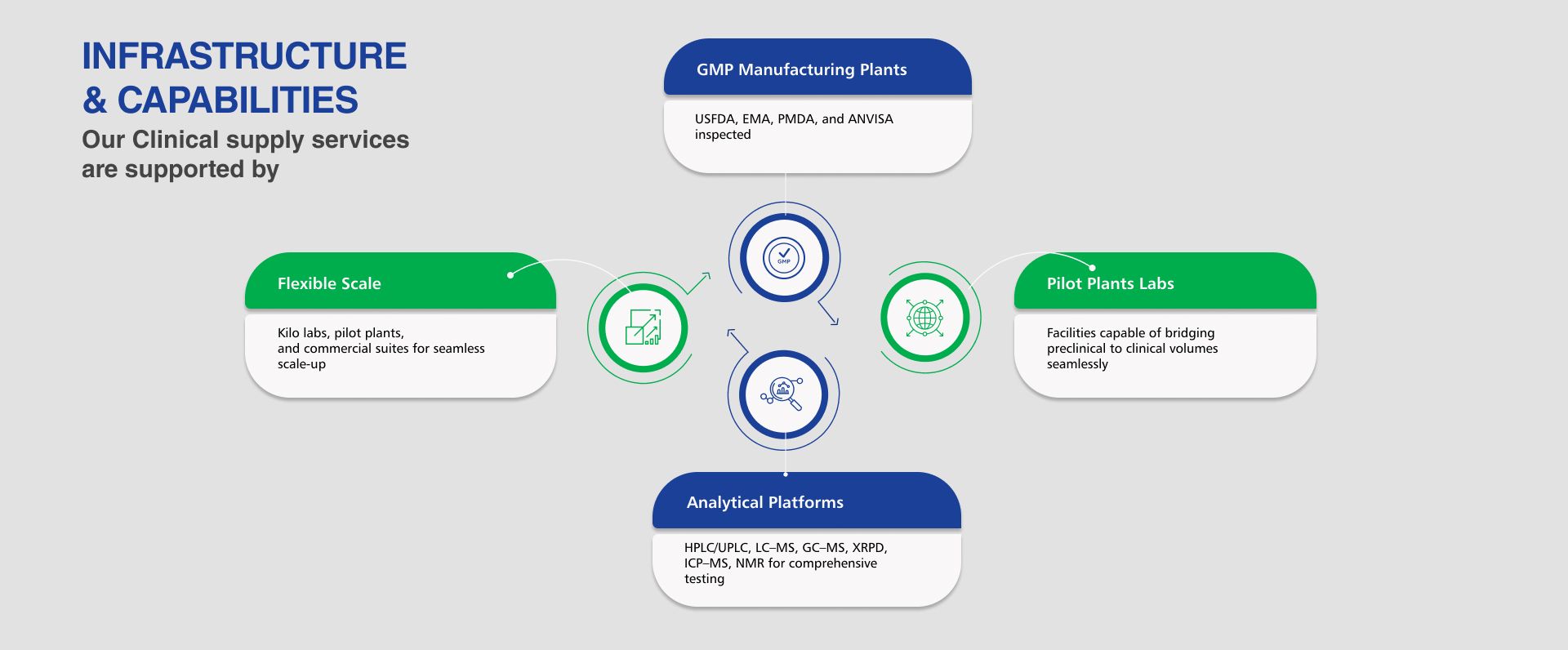
GMP Manufacturing for Phase I–III
- Small- to medium-scale GMP manufacturing of APIs for all clinical phases
- Batch sizes tailored to study size and protocol requirements
- Multi-site capability for redundancy and uninterrupted supply
Phase-Appropriate Control Strategies
- Specifications adapted to Phase I, II, or III requirements
- In-process controls and release testing aligned with regulatory expectations
- Provisional impurity acceptance for early phases, tightened controls for late-stage
Analytical Validation & Documentation
- Method validation to ICH Q2 standards
- CoAs, validation protocols, and full analytical reports provided
- Ready-to-submit documentation supporting INDs, NDAs, and global regulatory filings
Stability Programs
- ICH-compliant long-term, accelerated, and stress stability studies
- Zone-specific stability testing to meet regional requirements
- Shelf-life assignment and ongoing monitoring for clinical batches
Packaging, Labeling & Logistics
- Clinical batch packaging and GMP-compliant labeling
- Cold-chain logistics and validated shipping for temperature-sensitive APIs
- Transparent supply chain monitoring and tracking
WHY LMS?
- End-to-end continuity Preclinical → clinical → commercial within one CDMO network
- Regulatory excellence 100+ successful inspections across global markets
- Clinical phase agility Specifications and controls adapted to Phase I–III needs
- Complex chemistry expertise Ability to supply APIs involving HPAPIs, hormones, macrolides, and controlled substances
- Project management Dedicated client liaisons ensure transparent communication and milestone tracking
FAQs
What batch sizes can LMS manufacture for clinical trials?
How do you manage regulatory documentation for clinical supplies?
Can LMS handle APIs requiring special containment (e.g., HPAPIs)?
How do you ensure continuity from clinical to commercial supply?
Can LMS manage global distribution of clinical APIs?
Close

