INTEGRATED OFFERINGS
Lupin Manufacturing Solutions (LMS) delivers fully integrated CDMO offerings, enabling seamless progression from building blocks and key intermediates to drug substance and drug product under one trusted partner. Our unified platform brings together chemistry, formulation, analytics, scale-up, GMP manufacturing, packaging, and distribution, reducing complexity and development risk.
With harmonized quality systems, regulatory strength, and robust project governance, we ensure continuity, efficiency, and speed across every milestone. LMS supports small molecules, complex APIs, peptides, and oncology programs, backed by advanced containment and compliance capabilities. Partner with LMS for end-to-end integration that accelerates development, strengthens reliability, and shortens early development-to-market timelines.
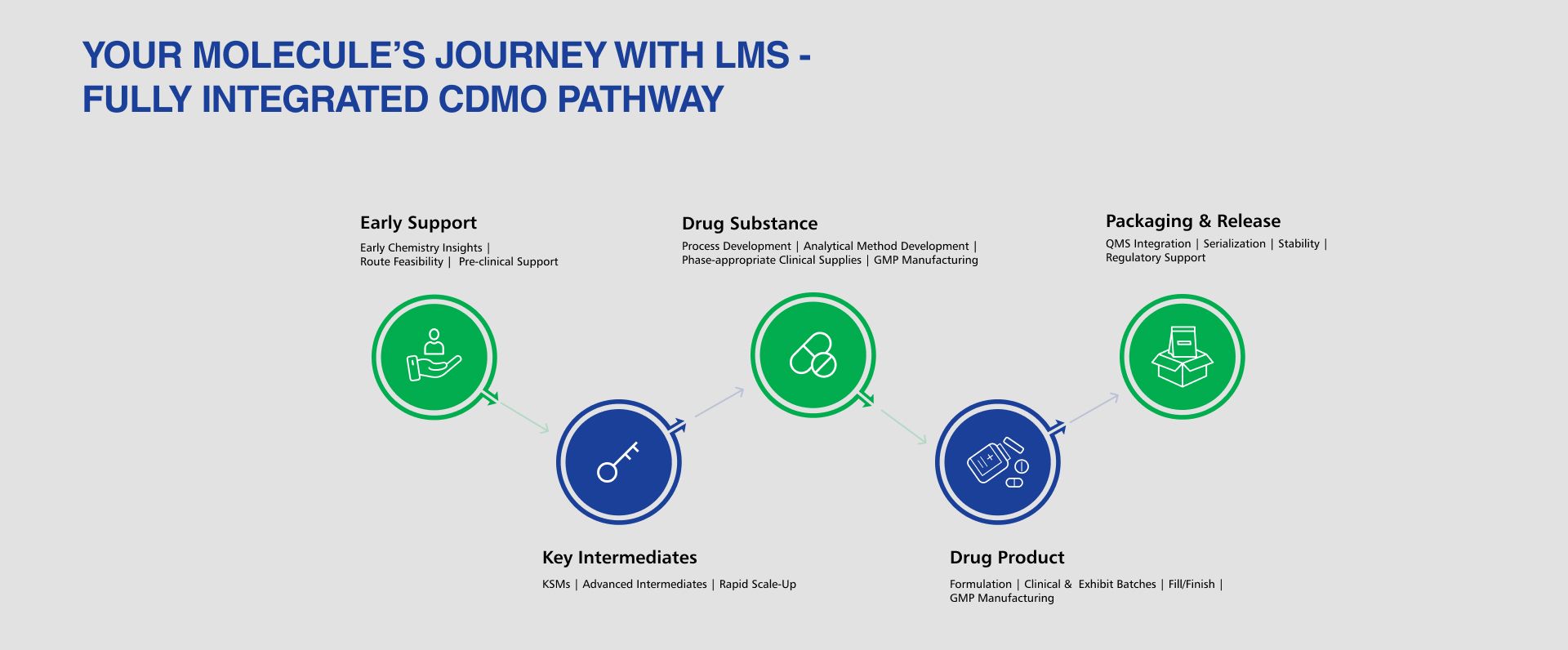
END-TO-END, SEAMLESS, SCALABLE EXPERTISE
- Early Chemistry to Key Intermediates Intelligent route design, rapid scale-up, and reliable production of complex intermediates.
- Drug Substance Development & API Manufacturing Process development, optimization, validation, and commercial-scale cGMP manufacturing.
- Drug Product Development & Commercialization Formulation, scale-up, clinical supplies, exhibit batches, and commercial multi-dosage manufacturing.
- Connected Tech Transfer Unified documentation, consistent analytics, and synchronized teams ensure smooth transitions across every stage.
- Quality & Regulatory Continuity One integrated quality framework supporting global filings minimizing variability and maximizing success.
WHY LMS?
- One Dedicated Partner All data, decisions, and process history stay within a single ecosystem.
- Faster Progression Through Development No rework, no repeated characterization, no multi-site onboarding.
- Lower Cost & Higher Predictability Integrated planning reduces development drag, materials waste, and logistical overhead.
- Consistent Quality Across All Stages One quality system → one analytical strategy → one validated workflow.
- Designed for Scale and Commercial Success From gram-scale feasibility to hundreds of tons of API and millions of tablets.

