PRE-FORMULATION DEVELOPMENT SERVICES
Pre-formulation is the foundation of successful drug product development. At LMS, we conduct comprehensive physicochemical, stability, and excipient compatibility studies to understand API behavior early and guide formulation decisions with precision. Using advanced characterization tools and API-sparing strategies, our team helps innovators de-risk development, shorten timelines, and ensure scalability.
OUR CAPABLITIES
Physicochemical Profiling
- Solubility, pKa, hygroscopic assessments
- Polymorph screening & solid-state characterization
- Particle size distribution & morphology studies
- API degradation pathway understanding
Excipient Compatibility & Stability Studies
- API–excipient interaction evaluations
- Thermal, photo stability & stress testing
- Compatibility reports to guide formulation selection
API Behavior & Manufacturability Insights
- Flowability & compressibility assessments
- Early identification of manufacturing challenges
- Data-driven recommendations for dosage form selection
Formulation Strategy Development
- Bioavailability enhancement pathways
- Selection of enabling technologies
- Rapid feasibility studies to reduce re-formulation risk
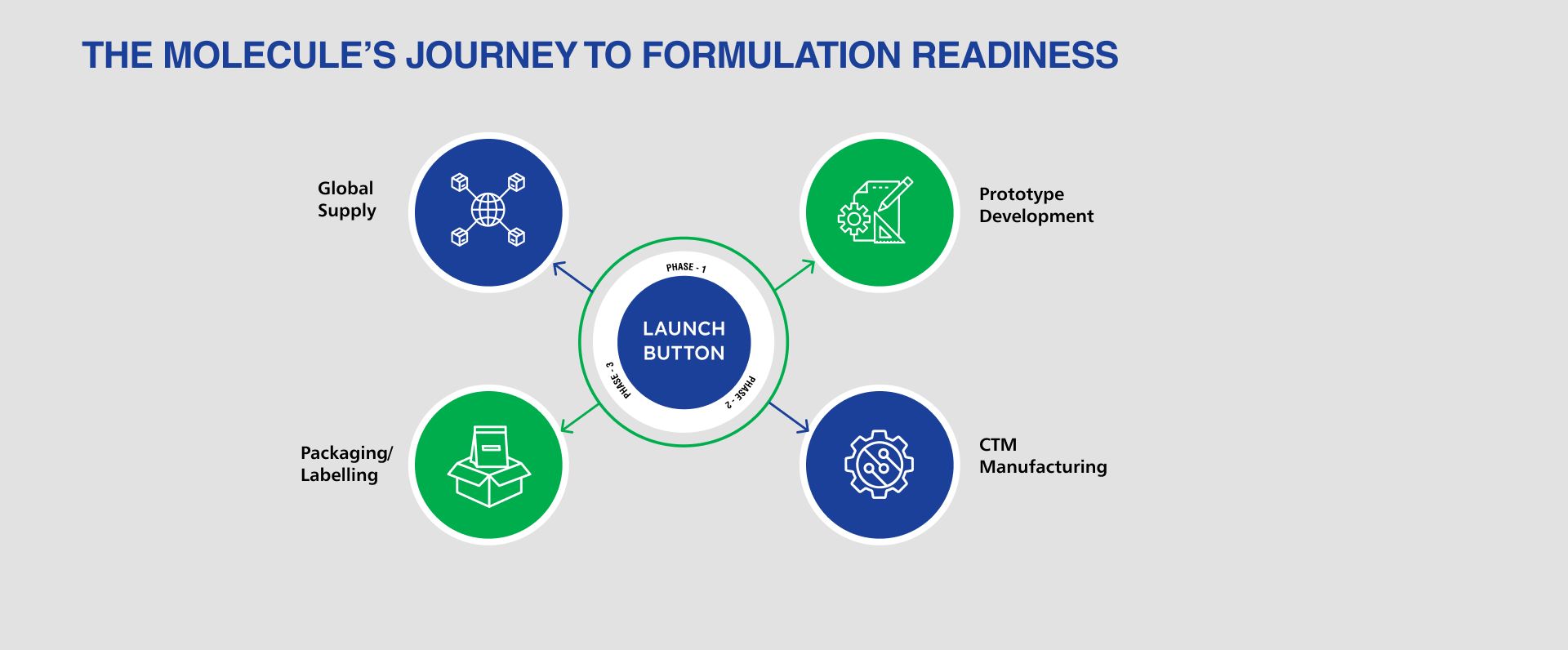
WHY LMS ?
- Comprehensive suite of analytical & characterization tools
- Strong linkage between pre-formulation and downstream formulation/scale-up
- API-sparing strategies reduce material consumption & cost
- Experienced scientists with exposure to global regulatory expectations
- Early risk identification improves speed to IND/NDA
FAQs
When should pre-formulation studies begin?
Do you support low-API availability programs?
What dosage forms do these studies support?
Close

