FORMULATION DEVELOPMENT SERVICE
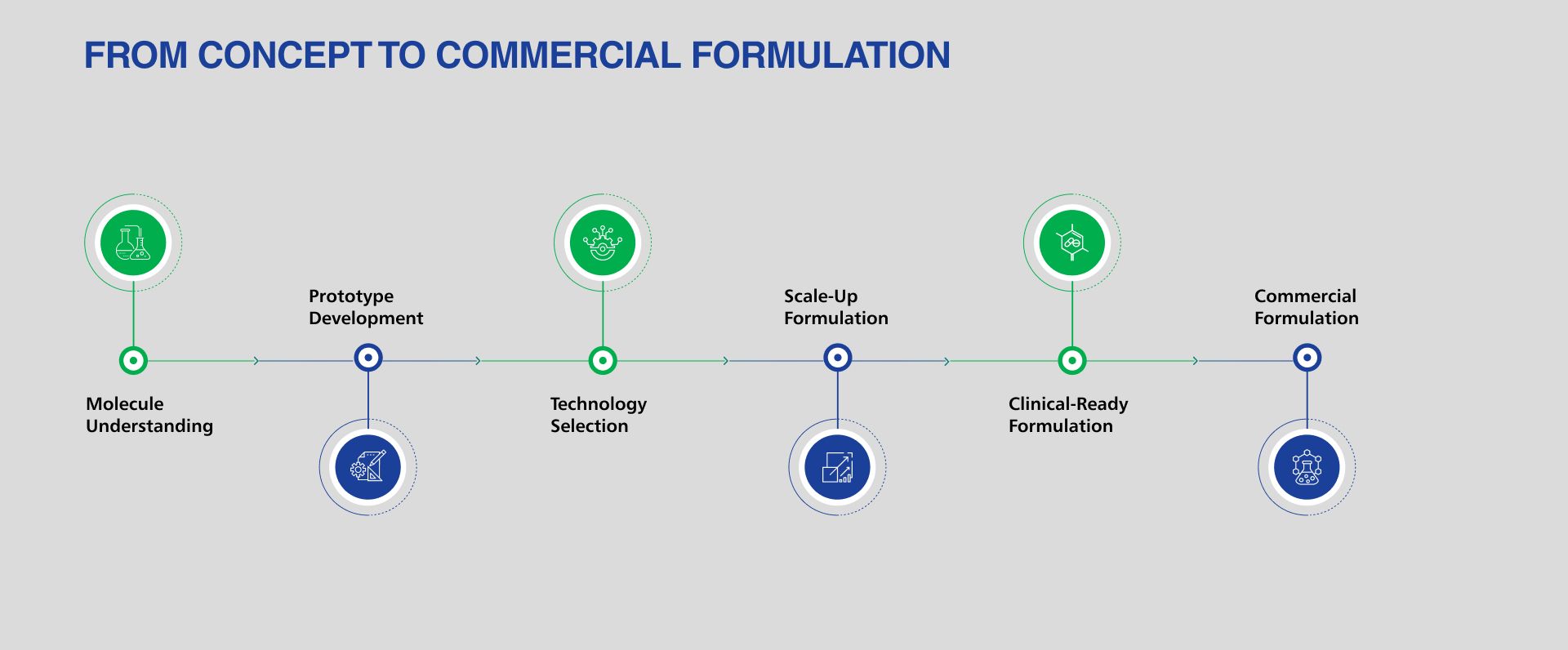
LMS delivers formulation development across a wide range of dosage forms from oral solids and injectables to inhalation, ophthalmic, topical and liquid products. Leveraging advanced drug delivery technologies, regulatory-aligned design and manufacturing insight, we build scalable formulations optimized for clinical and commercial success.
OUR CAPABILITIES
Oral Solid Dosage Forms
- Tablets and Capsules
- Chewables, sachets, fixed-dose combinations
- Hormonal & oncology (OEB 4) capability
- Development using wet/dry granulation, fluid-bed processing, coating
Parenteral / Injectable Formulations
- Liquid & lyophilized vials
- Prefilled syringes, cartridges
- Terminal sterilization support
- Device assembly for auto-injectors & pen devices
Ophthalmic Formulations
- Sterile solutions, suspensions, emulsions, gels
- Nanonization & homogenization processes
Respiratory Products
- MDI formulations with high-speed filling lines
- DPI powder formulation and capsule filling
- Nasal sprays, multi-dose & unit dose
Topical & Dermatological Forms
- Creams, ointments, lotions, gels, sprays
- Steroid & corticosteroid expertise
Oral Liquids & Suspensions
- Solutions, syrups, dry syrup blends
- Powders for suspension
Advanced Formulation Technologies
- Amorphous solid dispersions
- Lipid systems
- Nano-milling / Micronization
- Cyclodextrin complexes
WHY LMS?
- Full spectrum of dosage forms across small molecules & complex products
- Regulatory-compliant processes aligned with USFDA, EMA, PMDA
- Scalability built into early-stage design
- Access to specialized infrastructure (high potent, sterile, ophthalmic, inhalation)
- Proven track record of commercializing formulations for global markets
FAQs
Do you work with early-stage molecules?
Can you reformulate an existing product?
What data do you need to start?
Close

