CLINICAL DEVELOPMENT SERVICE
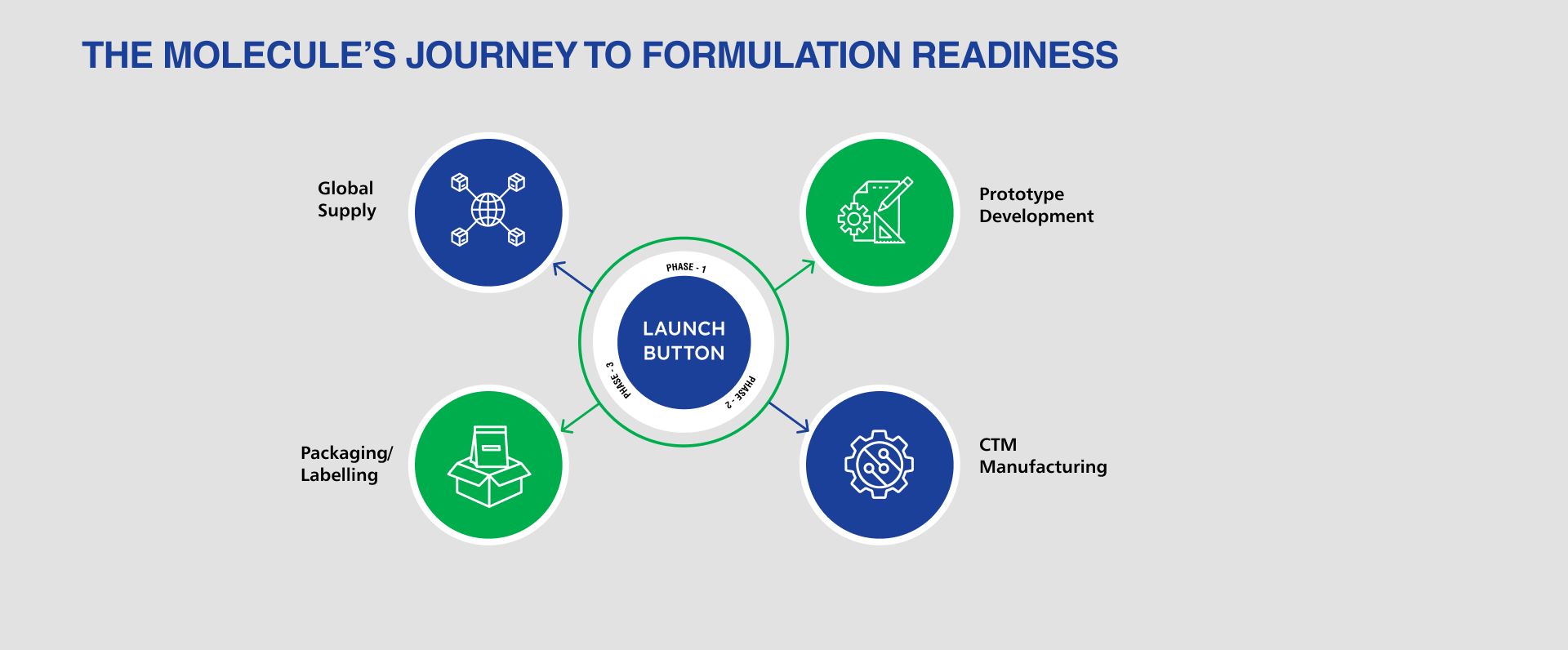
LMS provides flexible, GMP-compliant manufacturing of clinical trial materials (CTM) from Phase I through Phase III. Our API-sparing, phase-appropriate approach ensures consistency, regulatory readiness, and timely delivery of global clinical supplies.
OUR CAPABILITIES
Early-Phase Formulation Development
- Prototype development
- Screening batches using minimal API
- Rapid feasibility studies
GMP Clinical Supply Manufacturing
- Active drug & placebo manufacturing
- Blinded/controlled batches
- Packaging & labelling for global trials
Flexible Batch Sizes
- Small-scale for Phase I
- Medium-sized batches for Phase II/III
- Consistency across batches ensured
Stability & Regulatory Support
- IMPD/CMC documentation
- Stability studies under ICH guidelines
- Comparator sourcing assistance
WHY LMS?
- Speed Rapid turnaround for early-phase clinical materials
- Quality GMP alignment with global expectations
- Flexibility Batch sizes tailored to study demands
- Integration Smooth transition to late-stage & commercial manufacturing
FAQs
Can you support blinded studies?
What documentation do you provide?
Close

