
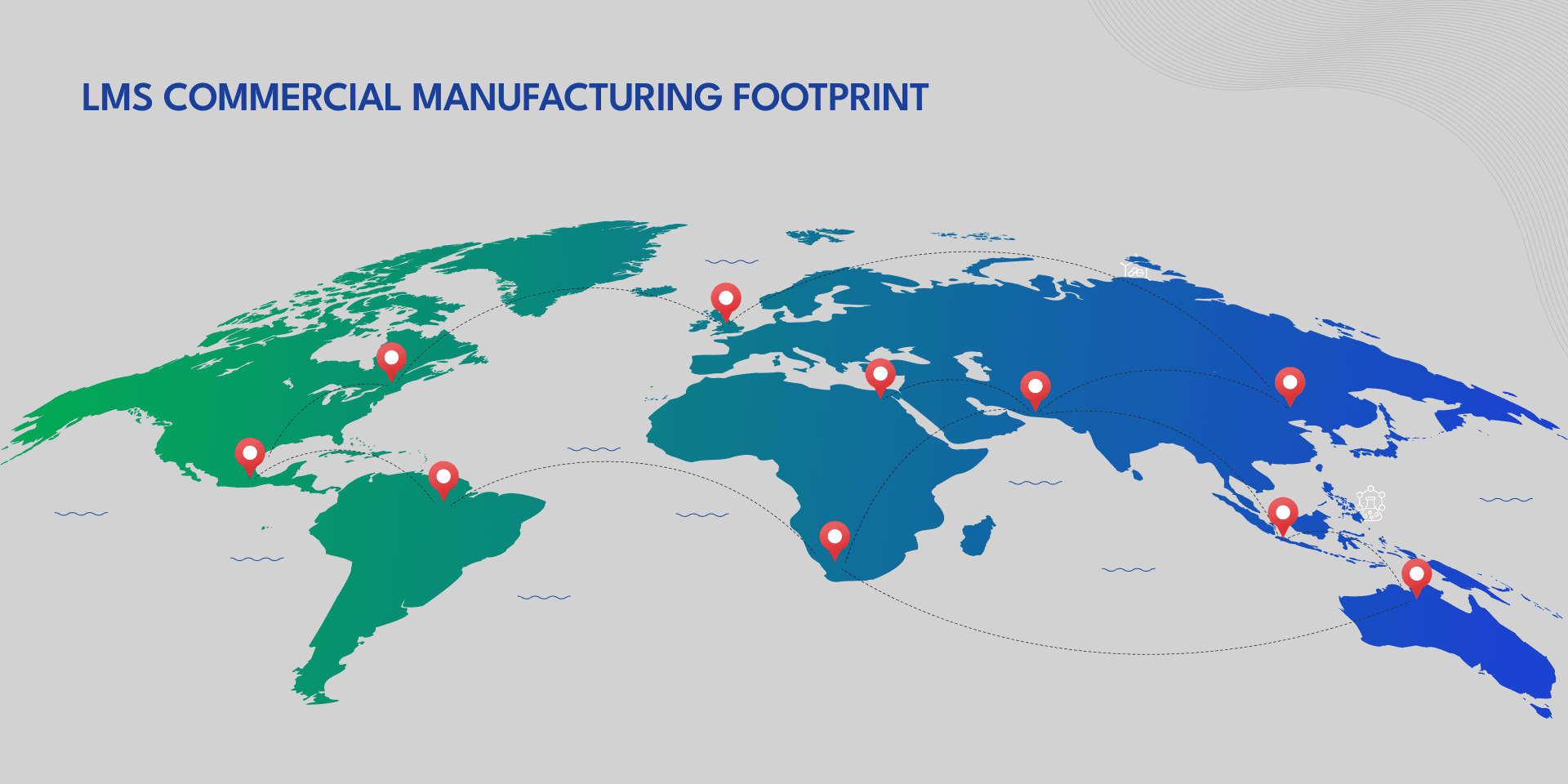
COMMERCIAL SUPPLY
LMS operates a global network of USFDA, EMA, MHRA, PMDA, TGA, WHO-approved facilities delivering high-quality commercial production across dosage forms. LMS also provides robust sterile fill-finish services for liquid and lyophilized injectables, supported by advanced aseptic environments and device assembly capabilities. Our robust infrastructure ensures reliability, scalability, and compliance for global supply.
OUR CAPABILITIES
Oral Dosages
- Oral Solids – Tablets, Capsules, Softgels
- Semi-solids – Topical creams, Gels, Lotion
- Oral Liquids – Solutions, Emulsions, Suspensions
Sterile Dosage Forms
- Liquid vials
- Lyophilized vials
- Prefilled syringes
Drug-Device Combinations
- Auto-injectors
- Pen-device systems
- Cartridge-based dose delivery
WHY LMS?
- Proven track record across global geographies including USA, EMEA & APAC
- High-volume capacity for global demand
- Stringent quality systems & integrated analytical capability
- Expertise in complex & high-potent products
- Fully integrated supply chain from development → market
FAQs
Can you support global tech transfers?
Do you offer serialization?
Do you support low-volume clinical fills?
Can you handle complex injectables?
Can you handle high-potent or hormonal DPI formulations?
Close

